Recipes
Now that we are familiar with the concepts of the molecular model, let us dive into some examples.
Note
Molassembler ships with an experimental SMILES parser that, pending some bugfixes and some more implementation details, should achieve full openSMILES standard-compliacne and be stabilized in a future release.
It’s not yet part of the public API, but it can already do a lot, which is why you’ll see it in some of these examples.
Alanine enantiomers
Let’s get right into a Molecule example with
multiple stereopermutations. Here, we’re creating an alanine
Molecule from a SMILES string, creating its
enantiomer, generating a conformation for each molecule, and writing those to
separate files.
>>> alanine = io.experimental.from_smiles("C[C@@H](C(=O)O)N")
>>> has_multiple_stereopermutations = lambda p: p.num_stereopermutations > 1
>>> interesting_stereopermutators = list(filter(
has_multiple_stereopermutations,
alanine.stereopermutators.atom_stereopermutators()
))
>>> assert len(interesting_stereopermutators) == 1
>>> stereopermutator = interesting_stereopermutators[0]
>>> stereopermutator.num_stereopermutations
2
>>> # Figure out which is the 'other' stereopermutation
>>> other = 0 if stereopermutator.assigned == 1 else 1
>>> # Make a conformation of alanine and its enantiomer and write each to a file
>>> from copy import copy
>>> alanine_enantiomer = copy(alanine)
>>> alanine_enantiomer.assign_stereopermutator(stereopermutator.placement, other)
>>> def write_conformer(mol, filename):
result = dg.generate_random_conformation(mol)
assert not isinstance(result, str)
io.write(filename, mol, result)
>>> write_conformer(alanine, "alanine.mol")
>>> write_conformer(alanine_enantiomer, "alanine_enantiomer.mol")
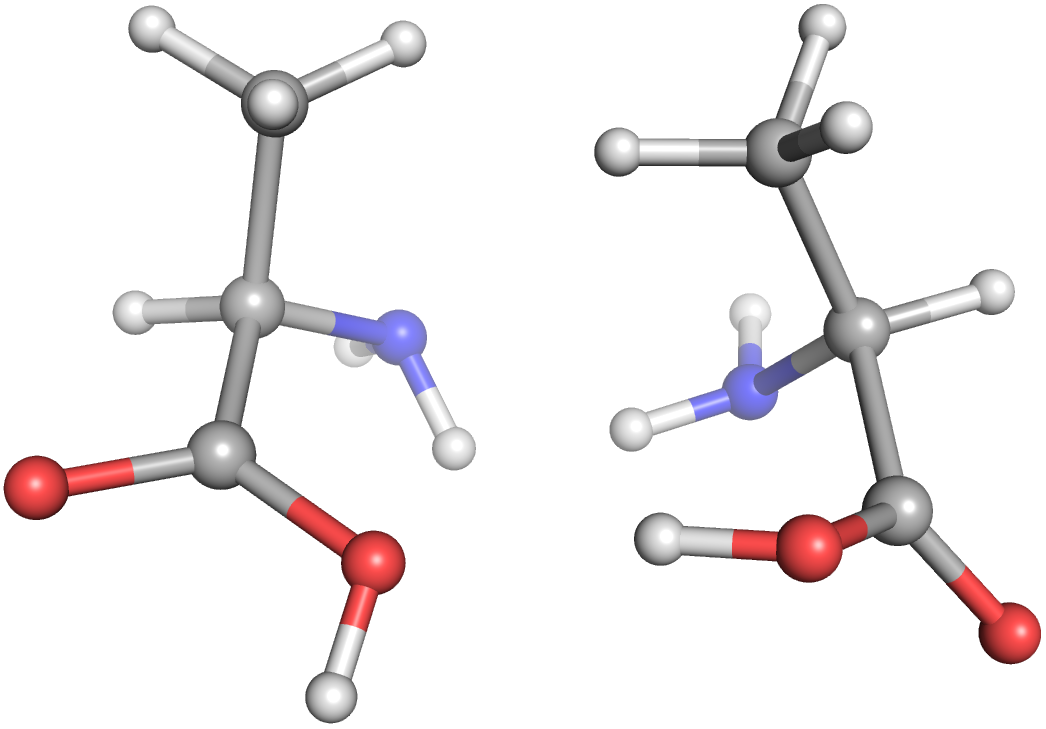
The two alanine enantiomers side-by-side generated by the previous script.
Ship-screw enantiomers
Let’s move on to a multidentate complex in which feasibility is relevant. We’ll consider the octahedral iron oxalate complex:
>>> shipscrew_smiles = "[Fe@OH1+3]123(OC(=O)C(=O)O1)(OC(=O)C(=O)O2)OC(=O)C(=O)O3"
>>> shipscrew = io.experimental.from_smiles(shipscrew_smiles)
>>> permutator = shipscrew.stereopermutators.option(0)
>>> assert permutator is not None
>>> permutator.num_stereopermutations # Number of abstract permutations
4
>>> permutator.num_assignments # Number of spatially feasible permutations
2
>>> permutator.index_of_permutation
2
>>> permutator.assigned
1
>>> for i in [0, 1]:
shipscrew.assign_stereopermutator(0, i)
result = dg.generate_random_conformation(shipscrew)
assert not isinstance(result, str)
io.write("shipscrew-" + str(i) + ".mol", shipscrew, result)